In Eastern Europe and Central Asia, the number of new HIV infections, AIDS diagnoses and AIDS-related deaths is rising steeply. These facts are inadequately known to the public at large, which is a good reason to ask for these parts of the world to be given more attention. The next large international biennial AIDS conference, to be held on 23–27 July 2018 in Amsterdam, will focus on developments in Eastern Europe and Central Asia

By Hans Houweling MD PhD

and Anke (J.J.) van Dam MD
AFEW International
HIV and AIDS in Eastern Europe and Central Asia: Pragmatism and Human Rights Versus Taboos
Summary
There is a widely-held impression that HIV infections and AIDS are now under control. After all, today there are effective treatment options which mean that HIV infections no longer need to lead to AIDS or death, and the spread of the virus has also been significantly curtailed. This picture holds reasonably true for the Western world, but it does not apply to sub-Saharan Africa, where new infections are still frequent and access to treatment is still problematic. And this positive image certainly does not apply to Eastern Europe and Central Asia, where the number of new infections, AIDS diagnoses and AIDS-related deaths is rising steeply. These facts are inadequately known to the public at large, which is a good reason to ask for these parts of the world to be given more attention. The next large international biennial AIDS conference, to be held on 23–27 July 2018 in Amsterdam, will focus on developments in Eastern Europe and Central Asia (EECA). This article will collate the available data on EECA in three sections: first the rise of HIV/AIDS in the 1980s, when the epidemic appeared to have left the Eastern bloc and the former Soviet Union comparatively untouched; then the situation today, with considerable diversity between countries; and lastly, an interpretation of these differences and some starting points for solutions, principally identified in the degree to which countries are able to set aside taboos surrounding sexuality and drug use and to adopt pragmatic solutions, such as safe sex and harm reduction campaigns for people who use drugs (for instance, the provision of clean needles and syringes, and methadone substitution therapy). A coherent programme of advocacy, human rights, prevention and care will be needed to turn the tide. On the basis of these starting points, AFEW International has drawn up programmes for key groups in six EECA countries: Georgia, Kazakhstan, Kyrgyzstan, Ukraine, Russia and Tajikistan.
The prelude: AIDS not a ‘Western disease’ after all?
In the former Eastern bloc and Soviet Union no rise in HIV infections or AIDS were observed before the end of the 1980s, while in the West the incidence of both was reported as rising rapidly. HIV infection was therefore regarded there as a Western disease spread by prostitutes, homeless people and homosexuals. In 1986 Anatoly Potapov, the Russian Minister of Public Health, called AIDS ‘a Western disease’, adding: “We don’t have the foundation for this infection spreading since Russia doesn’t have drug addicts and prostitution.”[1] AIDS was indeed rare in Eastern Europe at that time, compared to the Western world; in 1988 the total number of AIDS patients per million inhabitants (cumulative incidence) was 0.3 in Bulgaria, 1.3 in Hungary and 0.1 in the Soviet Union, compared with 20-40 per million in most Western European countries and as many as 62.5 in Denmark, 87.7 in France and 91.7 in Switzerland. The USA, with 323 per million, was recording even higher incidences.
The first case of AIDS was registered in the Soviet Union only in 1988. In Odessa (now the Ukraine) the first case of AIDS was also reported in late 1988; a baby, who had died shortly before. The baby’s mother had been infected through sexual contact.
Blood screening for HIV infection had been made available in the Soviet Union in 1987, and the first HIV infection case was quickly found: a man who had worked for many years in Africa. Within a year, 25 of his sexual partners were discovered to be HIV-infected.[2]
Hospital infections
Generally speaking, HIV infection and AIDS were therefore comparatively rare in Eastern Europe for some time. There were exceptions, however. In the 1980s about 10,000 children living in orphanages and hospitals in Romania were infected via unscreened blood transfusions and the re-use of unsterilized instruments.[3] [4] [5] As recently as 1998, 90% of Romanian AIDS patients were children; in that year Romania was responsible for two-thirds of all AIDS cases in Eastern Europe (4725 out of 7226).[3] In Elista, the capital of the southern Soviet republic of Kalmykia, another, smaller outbreak of hospital HIV infections was identified in 1988. The first cases included a female blood donor and her child.[6] Research revealed that the child had become infected in the local paediatric hospital, and follow-up research in that hospital showed that 75 children and four mothers had been infected. One of the children was the child of an HIV-positive mother and a father who had developed AIDS in the Congo. Infection prevention measures in the hospital were poorly implemented. Syringes were re-used without interim sterilizations; only the needles were replaced. A number of these children were transferred to Volvograd, Stavropol and Rostov-on-Don, resulting in a new wave of infections. By 1990, more than 270 children in Russia were found to have been infected with HIV.[2]
People who inject drugs
In 1995–1996 there were HIV infection epidemics amongst drug users in a number of cities in the former soviet republics: in the south in Odessa, Mykolayiv (Ukraine) and Krasnodar (Russia), and in the north in Kaliningrad (Russia) and several cities in Belarus. These epidemics affected tens of thousands of people.
Social change and increased vulnerability
It is notable that Central Europe has not experienced an HIV epidemic on the scale of that which occurred in Russia and Ukraine. The former Yugoslavia was one of the countries where the AIDS epidemic arrived early, especially amongst people who inject drugs (PWID). In 1992, the seroprevalence of HIV infection amongst PWID in Belgrade treatment programmes was as high as 44%.[7] In Poland, too, HIV started spreading comparatively early; between 1988 and 1989 the prevalence of HIV infection amongst PWID in a national sample survey rose from 1% to 9%. Amongst visitors to a detox clinic in Warsaw, this prevalence rose to 46% in 1993.[8] [9] The incidence of HIV and AIDS later stabilized in Poland and the countries of the former Yugoslavia. In Slovakia, the Czech Republic, Slovenia and Hungary the prevalence of HIV infection amongst PWID and other population groups remained low.*
The outbreaks of hospital infections in Romania and Elista had already demonstrated that the Eastern bloc was not immune to HIV infection and AIDS. The high rates of spread amongst injecting drug users in some Eastern European countries has to do with the far-reaching social changes that came in the wake of the collapse of the Soviet Union. Societies in Eastern Europe and Central Asia (EECA) had traditionally been characterized by strong social control, a low prevalence of injecting drug use, strict sexual norms, and the illegality of homosexuality. This, together with the closed, totalitarian Soviet system, ensured that AIDS appeared only sporadically until late in the 1980s. The collapse of the Soviet Union and its social structures and health care systems was followed by a steep rise in income inequality, ideological shifts, the breakdown of social values, and dire poverty amongst large sections of society. The results included changes in sexual morality and the growth of migrant labour, prostitution, and also injecting drug use, stimulated by the return of soldiers from Afghanistan and drug smuggling from Central Asia. The rapid growth in HIV infection, other STDs and other infectious diseases in parts of the former Soviet Union at the end of the 20th century has to be seen against this background. The fight against AIDS in the countries concerned, however, has long remained based on the approaches that prevailed in the time of the Soviet Union, particularly large-scale population screening. There has traditionally been little attention given to primary prevention.
The countries of Central Europe were more open to Western influences and therefore encountered HIV infections earlier than did Russia, Ukraine and the other Soviet republics. While the countries of Central Europe also experienced social upheaval and poverty, it was not to the same degree as in Russia and Ukraine. A number of these countries – such as Poland, the Baltic states and the Czech Republic – have since undergone favourable economic development. There has been no large-scale AIDS epidemic in these countries, possibly also because the campaign against AIDS has benefited more from the lessons learned in Western countries and is more strongly geared towards primary prevention than in the countries of the former Soviet Union.
All in all, then, HIV infection and AIDS are less exclusively a ‘Western disease’ than was long believed in the Eastern bloc.
Denial doesn’t help
In Africa, Eastern Asia, Western and Central Europe, Latin America, North America and the Caribbean, the number of people dying of AIDS is currently falling. By contrast, the numbers dying of AIDS in the EECA countries, the Middle East and in North Africa are rising (Figure 1).
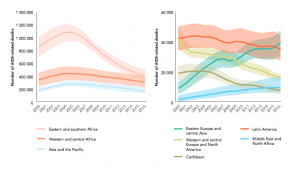
Figure 1: Deaths from HIV infection and AIDS by region, 2000–2016. Source: UNAIDS, 2017 estimates.[10]
The number of new HIV infections in the EECA countries is also exhibiting an alarming growth (Figure 2).
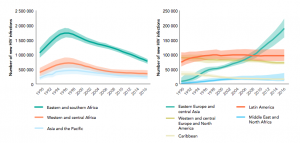
Figure 2: New HIV infections, all ages, by region, 1990–2016. Source: UNAIDS, 2017 estimates.[10]
Figures 1 and 2 are based on model estimates. However, since 2006 actual diagnoses of HIV infections have been centrally registered by the European region of the World Health Organization (WHO); this region also comprises all the countries of the former Soviet Union. Figure 3 shows the strong rise in HIV infections being seen in the eastern part of this region.** These registered numbers have not been corrected for under-diagnosis (the diagnosis is not made) and under-reporting (the diagnosis is made but not reported), and for this reason it is an underestimate of the actual situation. Although these data are naturally subject to distortions arising through differences between countries in the quality of the registration procedure, they nevertheless give a clear indication of the scale of the HIV epidemic in the region and of the large differences being seen between different countries.
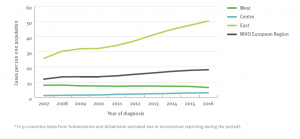
Figure 3: Number of new HIV diagnoses per 100,000 of the population, by year of diagnosis, WHO European region, 2006–2016. Source: European Centre for Disease Prevention and Control/WHO Regional Office for Europe, 2017.[11]
These general estimates conceal wide differences between countries. For example, Figure 4 shows the number of HIV diagnoses as before, but omits the data for Russia. This demonstrates that a large proportion of the growing HIV epidemic in the Eastern subregion is taking place in Russia. The number of HIV infections is also comparatively large in Ukraine. Taken together, in 2016 Russia and Ukraine were together responsible for 73% of all HIV infections in the European region, and for 92% of all HIV infections in the Eastern subregion.
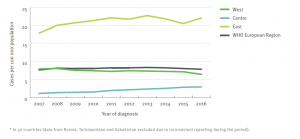
Figure 4: Number of new HIV diagnoses per 100,000 of the population, by year of diagnosis, WHO European region, 2006–2016 (excluding Russia). Source: European Centre for Disease Prevention and Control/WHO Regional Office for Europe, 2017.[11]
Table 1 shows the number of newly-diagnosed HIV infections per 100,000 of the population for all the countries of the Eastern subregion in 2016, alongside the total number of HIV infections in that country since registration began. For the subregion as a whole, in 2016 the number of reported HIV infections was 50.2 per 100,000 inhabitants.*** Women make up about 40% of this total, a significantly higher proportion than in the Central European (26%) and Western European (28%) WHO subregions.
Eastern Europe and Central Asia therefore together form one of the few regions in the world where the number of deaths from AIDS and the number of new HIV infections continues to rise. The number of HIV infections in the population is particularly high in Russia, Ukraine, Uzbekistan, Belarus and Moldova, and also relatively high in Georgia and Kazakhstan. Whereas the HIV epidemic in EECA countries originally affected people who inject drugs most of all, today heterosexual men and women are responsible for the majority of new infections (see Figure 5). Clearly, simply denying that the problem exists is not an effective approach to dealing with HIV and AIDS.
Table 1: The number of newly-diagnosed HIV infections per 100,000 of the population in 2015, and the total number of reported newly-diagnosed HIV infections in the period since registration began, in the countries of Eastern Europe and Central Asia (together the WHO’s Eastern subregion). Source: European Centre for Disease Prevention and Control/WHO Regional Office for Europe, 2017.[11]
| Country |
Total number since registration began* |
Number in 2016 per 100,000 inhabitants |
| Eastern Europe |
|
|
| Albania |
1007 |
4.4 |
| Bosnia-Herzegovina |
274 |
0.6 |
| Estonia |
9492 |
17.4 |
| Latvia |
6972 |
18.5 |
| Lithuania |
2749 |
7.4 |
| Macedonia |
157 |
1.4 |
| Moldova |
11021 |
20.5 |
| Montenegro |
228 |
5.4 |
| Ukraine |
246846 |
33.7 |
| Russia |
1114815** |
70.6 |
| Serbia |
3590 |
2.0 |
| Belarus |
22218 |
25.2 |
| Central Asia |
|
|
| Armenia |
2550 |
9.9 |
| Azerbaijan |
6185 |
5.6 |
| Georgia |
6131 |
18.1 |
| Kazakhstan |
29564 |
16.3 |
| Kyrgyzstan |
7170 |
12.5 |
| Uzbekistan*** |
24018 |
– |
| Tajikistan |
8748 |
12.0 |
| Turkmenistan*** |
2 |
– |
| Total in Eastern Europe and Central Asia |
1503737
|
50.2 |
* The starting year of registration can differ between countries. ** Apart from the year 2010, Russia has provided no official figures to the ECDC/WHO Regional Office for Europe. The figures given in this table for Russia are derived from the Russian Federal AIDS Centre.[12] *** No figures have been provided by Uzbekistan or Turkmenistan in recent years.
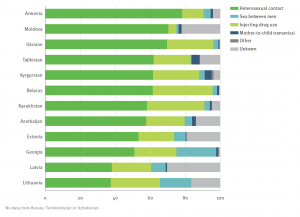
Figure 5: New HIV infections by country and risk factor, Eastern subregion, 2016 (n=24641). Source: European Centre for Disease Prevention and Control/WHO Regional Office for Europe, 2017.[11]
Transition and taboo
The scale of the HIV/AIDS epidemic in the EECA region was small until the end of the 1980s. Its spread since then has turned out to depend on contacts with countries in which the epidemic had already taken on a considerable scale (including Western European countries), social decay and the associated changes after the break-up of the Soviet Union, and the degree to which an effective public health response was achieved.
The collapse of the Soviet Union led to an enormous growth in one of its classic examples of Western decadence: drug use. An estimated 1% of the adult population of Central Asia uses intravenous drugs. Along drug trade routes, this figure can rise to 10%.[13] The Soviet model of strongly centralized and hierarchical health care was completely unprepared for the consequences of sweeping social change. Although surveillance was intensified in many countries, very limited numbers of prevention programmes were successfully instated. The EECA countries turned out to be very different in their capacity to adapt to all the changes. Most of these countries still have strong taboos surrounding sexuality and drug use – taboos which form a serious impediment to public health education, safe sex campaigns, and pragmatic, demonstrably effective ‘harm reduction’ solutions for drug users, such as needle exchange programmes and methadone substitution therapy. Uzbekistan, Russia and Turkmenistan have no official needle exchange programmes, and methadone substitution therapy in these countries is actually illegal. In countries where needle exchange and methadone provision programmes do exist, their availability is generally limited to a few cities. Many drug users are therefore never reached. The taboos surrounding sexuality and drug use also lead to many other problems, with drug users and sexual minorities subject to serious discrimination and stigmatization. Human rights, especially those of marginalized groups, are not respected. People in key groups and those actually living with HIV are only marginally involved in the design of information campaigns, treatment programmes and legislation, and this renders much of the fight against HIV infection ineffective. Following the example of the Soviet Union, health care is still predominantly organized along vertical lines: there is no consultation, for instance, between the doctor treating the HIV infection and the tuberculosis specialist, let alone an addiction specialist. The health care system puts doctors before patients; it is ‘provider-centred’ rather than ‘client-centred’. In many EECA countries HIV tests are seldom available, and the confidentiality of their results is often insecure. Access to HIV medicines is problematic. There is widespread ignorance about HIV infection and its treatment, and there are many myths about antiretroviral drugs (ARVs) which encourage people to avoid treatment. All in all, access to health care is very limited for those infected with HIV, and the UNAIDS ‘90-90-90’ targets for the region are unlikely to be achieved. The UNAIDS programme has set itself three aims to achieve by 2020: 90% of the HIV infections in the population will have been identified, 90% of those known to be HIV-infected will be undergoing treatment with antiretroviral drugs, and in 90% of these cases the virus will have been suppressed to the point that its presence is no longer detectable and the patient is no longer contagious. These current estimated percentages for Eastern Europe and Central Asia are well below global averages (see Figure 6).[13]
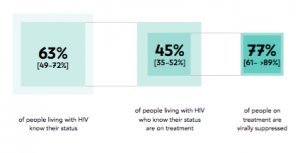
Figure 6: The percentages of 1) HIV carriers who are aware they are infected, 2) those who are aware that they carry HIV and who are also undergoing treatment, and 3) those undergoing treatment in whom the virus is no longer detectably present; Eastern Europe and Central Asia [with uncertainty intervals]. Source: UNAIDS 2017 estimates.[13]
AFEW International
A coherent programme of advocacy, human rights, prevention and care will be needed to turn the tide. On the basis of these starting points, AFEW International has drawn up programmes for key groups in six EECA countries: Georgia, Kazakhstan, Kyrgyzstan, Ukraine, Russia and Tajikistan.
AFEW International is an international network of civil society organisations that is dedicated to improving the health of key populations. With a focus on Eastern Europe and Central Asia (EECA), AFEW strives to promote health and increase access to prevention, treatment and care for public health concerns such as HIV, TB, viral hepatitis, and sexual and reproductive health and rights. AFEW seeks to do this by advocating for human rights for key populations and protecting their rights to health, decreasing the stigma of HIV/AIDS by providing information to community leaders and creating a supportive environment, utilising innovative strategies to promote healthy behaviours, and engaging communities in developing participatory approaches. The vision of AFEW International is a world in which HIV does not destroy lives through stigma and discrimination and where all people, regardless of their HIV status, have access to healthcare and other services that give them the opportunity to achieve their full potential.
AFEW strengthens the capacity of professionals in the region through the adoption of effective methods of HIV prevention, treatment, care and support given the specific circumstances in their countries. AFEW sees its role as providing assistance in such a way that appropriate action is taken, with the objective of strengthening local capacity and ensuring that the final responsibility remains with those in the society itself. Learning from best-practice experiences elsewhere in the world and from knowledge gained in EECA, AFEW develops innovative tools and approaches adapted to a specific context. AFEW builds upon its own practical experience within the region, strives for ongoing improvements in its practices, and manages and transfers knowledge to the relevant stakeholders.
Acknowledgements
The authors thank Joost van der Meer MD PhD for his thoughtful comments and suggestions to an earlier version of this manuscript.
Footnotes
* Today’s partition between Western and Eastern Europe derives originally from the Great Schism of 1054, when the Roman Catholic church of the West and the Orthodox Christian church of the East permanently parted ways. However, the expansion of Russian influence from the late 19th century up until the Cold War and the geopolitical developments of recent decades have also left their traces. The borders between Western, Central and Eastern Europe are therefore still subject to debate. Until 1989, the countries of Central Europe were politically allied with the Soviet Union, forming the ‘Eastern bloc’. Since 1989 these countries have turned increasingly towards the West. Today the Baltic states form part of the European Union; as such they are often seen as not being part of Eastern Europe, despite their easterly geographical location. Serbia and Ukraine, although both overwhelmingly Eastern Orthodox in religious terms, are generally seen as belonging to either Central Europe or Eastern Europe. In this article, all former Soviet republics, together with Albania and parts of the former Yugoslavia (Bosnia-Herzegovina, Montenegro and the former Yugoslav Republic of Macedonia) are held to lie in the region of Eastern Europe and Central Asia (EECA).
** The 53 countries of the WHO European region were – on the basis of epidemiological considerations and in conformation with earlier reports on HIV/AIDS surveillance – divided into three geographical areas: 1) the subregion ‘West’ (23 countries: Andorra, Belgium, Denmark, Germany, Finland, France, Greece, Ireland, Iceland, Israel, Italy, Luxemburg, Malta, Monaco, the Netherlands, Norway, Austria, Portugal, San Marino, Spain, the United Kingdom, Sweden, and Switzerland); 2) the subregion ‘Centre’ (15 countries: Albania, Bosnia-Herzegovina, Bulgaria, Cyprus, Hungary, Croatia, Macedonia, Montenegro, Poland, Romania, Serbia, Slovenia, Slovakia, the Czech Republic, and Turkey); and 3) the subregion ‘East’ (15 countries: Armenia, Azerbaijan, Estonia, Georgia, Kazakhstan, Kyrgyzstan, Latvia, Lithuania, Moldova, Ukraine, Uzbekistan, Russia, Tajikistan, Turkmenistan, and Belarus).
*** For the purposes of comparison, in the Central subregion the number of reported HIV infections in 2016 was 2.9 per 100,000 inhabitants; in the Western subregion it was 6.2 per 100,000 inhabitants; and in the Netherlands it was 4.4 per 100,000 inhabitants.
Literature
[1] Cited in Alla Salkova. Not a ‘Western disease’ after all: How HIV appeared in the USSR. Internet: Russia Beyond, 3 November 2016: https://www.rbth.com/politics_and_society/2016/11/03/not-a-western-disease-after-all-how-hiv-appeared-in-the-ussr_644835 [consulted 27 March 2018].
[2] Alla Salkova. Not a ‘Western disease’ after all: How HIV appeared in the USSR. Internet: Russia Beyond, 3 November 2016: https://www.rbth.com/politics_and_society/2016/11/03/not-a-western-disease-after-all-how-hiv-appeared-in-the-ussr_644835 [consulted 27 March 2018].
[3] Hamers FF, Downs AM, Infuso A, Brunet J. Diversity of the HIV/AIDS epidemic in Europe. AIDS 1998; 12 (suppl. A): S63-70.
[4] Hersh BS, Popovici F, Apetrei RC, e.a. Acquired immunodeficiency syndrome in Romania. Lancet 1991; 338: 645-9.
[5] Hersh BS, Popvici F, Zolotusca L, e.a. The epidemiology of HIV and AIDS in Romania. AIDS 1991; 5 (Suppl. 2): S87-92.
[6] Pokrovsky VV, Eramova EU, Deulina MO, e.a. Vnutribol ‘nichnaia vspyshka VICh-infektsis v Eliste (An intrahospital outbreak of HIV-infection in Elista). Zhurnal Mikrobiologii Epidemiologii Immunobiologii 1990: 17-23.
[7] Kilibarda M. HIV infection among drug abusers in the Belgrade area. Bull Narc 1993; 45: 135-46.
[8] Hamers FF, Batter V, Downs AM, Alix J, Cazein F, Brunet JB. The HIV epidemic associated with injecting drug use in Europe: geographic and time trends. aids 1997; 11: 1365-74.
[9] Gromyko A. Sexually transmitted diseasee epidemic in eastern Europe: a call for help. Entre Nous 1996; 33: 7-8.
[10] Ending AIDS: progress towards the 90-90-90 targets. Genève: Joint United Nations Program on AIDS (UNAIDS), 2017. Internet: http://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017 [consulted on 14 November 2017].
[11] European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2017 – 2016 data. Stockholm: ECDC, 2016. Internet: https://ecdc.europa.eu/en/publications-data/hivaids-surveillance-europe-2017-2016-data [consulted on 13 February 2018].
[12] Russian Federal Scientific and Methodological Center for Prevention and Control of AIDS. Information note Spravka on HIV infection in the Russian Federation as of 31 December 2016. Moscow: Russian Federal Scientific and Methodological Center for Prevention and Control of AIDS; 2017 [cited in 11].
[13] UNAIDS Country reports on progress in implementation of the global response to HIV infection, 2012 for Kazakhstan, Kyrgyzstan and Uzbekistan, cited in DeHovitz J, Uuskula A, El-Bassel N. The HIV epidemic in Eastern Europe and Central Asia. Curr HIV/AIDS Rep 2014 DOI 10.1007/s11904-014-0202-3.